Abstract
MAJIC is a phase II trial of Ruxolitinib (RUX) vs Best Available Therapy (BAT) in polycythemia vera (PV) patients with resistance/intolerance to Hydroxycarbamide (HC). This analysis involved a primary comparison between the RUX and BAT arms for quality of life (QOL) durability over 60 months, with secondary comparisons of best QOL response within first year in complete hematologic responders (CR) vs a group of partial or no response (NR/PR). This is a unique analysis due to cross-over nature of prior studies.
Patients were stratified by treatment arm with either RUX or BAT. QOL was assessed over 0-60 months using the Myeloproliferative Neoplasms Symptom Assessment Form (MPN-SAF). MPN-SAF Total Symptom Score (TSS) was computed as the average of all completed items multiplied by 10 (scale 0‐100, higher score represents higher symptom burden). Estimates of change from baseline and between arm differences in change by timepoint were made using a linear mixed model with compound symmetry covariance structure, which included covariates for categorical time point, treatment arm, and the interaction between time point and treatment arm. The difference between arms in proportion of patients with best post-baseline TSS response of 50% or greater was testing using a Chi-square test.
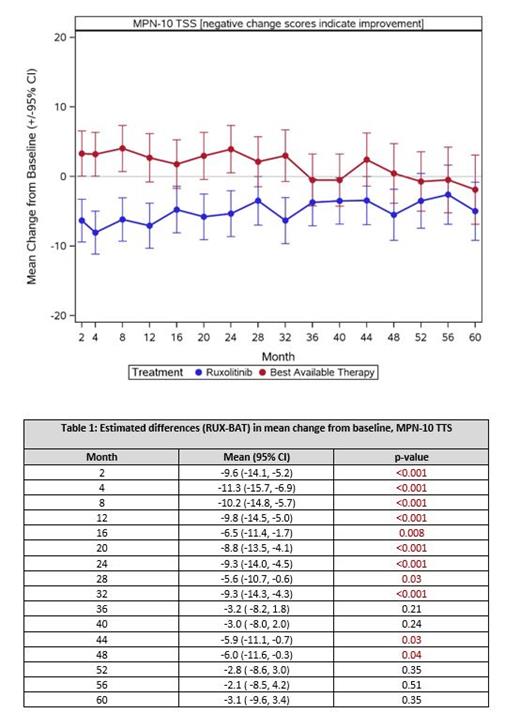
In this study, 147 of the 190 trial patients had started treatment and completed at least their baseline assessment and were included in the analysis with 39 patients completing through 60 months. Groups were comprised of 76 patients in the RUX group (31 females, 45 males, mean age 64.2 [SD 11.4]) and 71 patients in the BAT group (29 females, 42 males, mean age 64.6 [SD 11.5]). Symptom scores at baseline were similar between arms, with MPN-SAF weight loss the only statistically significant difference (BAT 0.7 [SD 1.71] vs RUX 1.7 [SD 2.83], p=0.02). Significant, durable improvements in TSS were noted in RUX patients with symptom improvements lasting a mean of 52 months. BAT patients experienced a worsening of their symptom burden with improvements back to baseline notable at 56 months (Figure 1). MPN-SAF TSS there were significant differences between treatment arms in change from baseline to months 2-32, 44, and 48 (all p<0.05). In all months the trend was in the direction of larger improvement in the RUX arm, with the point estimate for difference ranging from 2.1 (at month 56) to 11.3 (at month 4) with most prominent changes occurring between months 2 and 32. Of the 80 patients with MPN-SAF TSS scores at baseline and at least one post-baseline timepoint, 13/41 (31.7%) BAT and 24/39 (61.5%) RUX patients had TSS reduction of 50% or greater in at least one time point, which was statistically significant (p=0.008). In regard to specific symptoms, there was a statistically significant between arm difference seen in over 5 timepoints for fatigue, early satiety, night-sweats, itching, bone pain, and weight loss.
Comparing MPN-SAF TSS in Complete Response (CR n=51) vs Non-Responder/Partial Responders (NR/PR) (n=96) there was no difference at baseline in any of the measures at any time point. A possible limitation of this study is the potential for bias in which the patients who were lost to follow up could be different then the patients who remained in the study through all 60 months which would affect generalization, particularly at time points at the later years.
The novel findings of this investigation demonstrate that Ruxolitinib ameliorates the PV symptom burden in patients resistant or intolerant to HC in a robust durable manner over at least four years, while patients receiving BAT have worsening of their symptom burden over this same time period.
Mead: Novartis: Consultancy, Honoraria, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria. Yap: Celgene: Honoraria; Faron Pharmaceuticals: Honoraria. Scherber: Incyte Corporation: Current Employment, Current holder of stock options in a privately-held company. Knapper: Novartis: Consultancy, Research Funding, Speakers Bureau; Astellas: Ended employment in the past 24 months, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau. Drummond: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI: Membership on an entity's Board of Directors or advisory committees. McMullin: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: clinical trial support, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan: Research Funding, Speakers Bureau. Mesa: CTI: Research Funding; AOP: Consultancy; Celgene: Research Funding; Novartis: Consultancy; Gilead: Research Funding; Pharma: Consultancy; Promedior: Research Funding; Constellation Pharmaceuticals: Consultancy, Research Funding; CTI: Research Funding; Samus: Research Funding; Sierra Oncology: Consultancy, Research Funding; Genentech: Research Funding; Incyte Corporation: Consultancy, Research Funding; Abbvie: Research Funding; La Jolla Pharma: Consultancy. Harrison: CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Constellation Pharmaceuticals: Research Funding; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Corporation: Speakers Bureau; Sierra Oncology: Honoraria; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal